Fda Approved Vaccines 2019
WHO is working tirelessly with partners to develop manufacture and deploy safe and effective vaccines. Equitable access to safe and effective vaccines is critical to ending the COVID-19 pandemic so it is hugely encouraging to see so many vaccines proving and going into development.
Https Www Modernatx Com Covid19vaccine Eua Providers Storage Handling Pdf
104 rijen FDA has rigorous scientific and regulatory processes in place to facilitate development and ensure the safety effectiveness and quality of COVID-19 vaccines.

Fda approved vaccines 2019. Food and Drug Administration announced today the approval of Jynneos Smallpox and Monkeypox Vaccine Live Non-Replicating for the prevention of. FDA Approval - 5119. The rVSV-ZEBOV vaccine was approved for medical use in the European Union in November 2019 and in the United States in December 2019.
Catholic leader fueled lies about Covid-19 vaccines before ending up on ventilator CNN The US Food and Drug Administration on Monday granted full approval to the PfizerBioNTech Covid-19 vaccine. Two vaccine manufacturers have submitted applications for full approval of their COVID-19 vaccines but the FDA hasnt yet indicated when a decision will come. Food and Drug Administration announced today the approval of Ervebo the first FDA-approved vaccine for the prevention of Ebola virus disease.
And the number of days between the submission of the licensure application and the date of FDA approval. Figures - available via license. Pfizer-BioNTech COVID-19 Vaccine May 10 2021.
THe fDa Vaccine approVal process M ost people think that the government is watching out for them and when they are told that vaccines are safe and efective they believe it in part because they know that these products have been approved by the US Food and Drug Administration FDA. The percentage of newly licensed vaccines reviewed by VRBPAC. However most people also know little to.
The product name and trade name of vaccines licensed for use in the United States. ZabdenoMvabea was approved for medical use in the European Union in July 2020. Today the US.
FDA approval and VRBPAC review of vaccines for emerging diseases 2000-2019. The FDA expanded the emergency use authorization of the Pfizer-BioNTech COVID-19 Vaccine to include adolescents 12. Although this approval count falls short of CDERs record 59 approvals of 2018 it still.
The FDAs Center for Drug Evaluation and Research CDER approved 48 novel drugs in 2019 Table 1. To treat acute treatment of migraine with or without aura in adults Press Release Drug Trials Snapshot. The approval comes in a week prior to federal health officials earlier estimates that the agency would complete its review by Labor Day.
Action Date Submission Supplement Categories or Approval Type Letters Reviews Labels Patient Package Insert Note Url. Understanding How COVID-19 Vaccines Work Learn how the body fights infection and how COVID-19 vaccines protect people by producing immunity. Creative Commons Attribution-NonCommercial-NoDerivatives 40.
December 19 2019 The US. Approval Date FDA-approved use on approval date 48. 519 rijen Coronavirus Disease 2019 COVID-19 FDA Coronavirus Disease 2019.
FDA approves Dengvaxia the first vaccine approved for the prevention of dengue disease in people ages 9 through 16 who have laboratory-confirmed previous dengue infection and who live in endemic areas. The Food and Drug Administration on Monday granted full approval of the Pfizer COVID-19 vaccine becoming the first covid-19 vaccine to transition from an emergency authorization status to full FDA approval. Food and Drug Administration approved the first COVID-19 vaccine.
We analyzed the frequency of VRBPAC meetings and sessions. Methods Cross-sectional study of VRBPAC meetings convened and new vaccine licensure applications approved between January 1 2000 and December 31 2019. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine.
Also see the different types of COVID-19 vaccines that currently are available or are undergoing large-scale Phase 3 clinical trials in the United States.

Securing The U S Drug Supply Chain Oversight Of Fda S Foreign Inspection Program 12 10 2019 Fda

Why Two Vaccines Passed The Finishing Line In A Year And Others Didn T And A Month 12 Roundup Absolutely Maybe

The Fda Needs To Speed Up Full Approval Of Covid 19 Vaccines Physician Says Npr
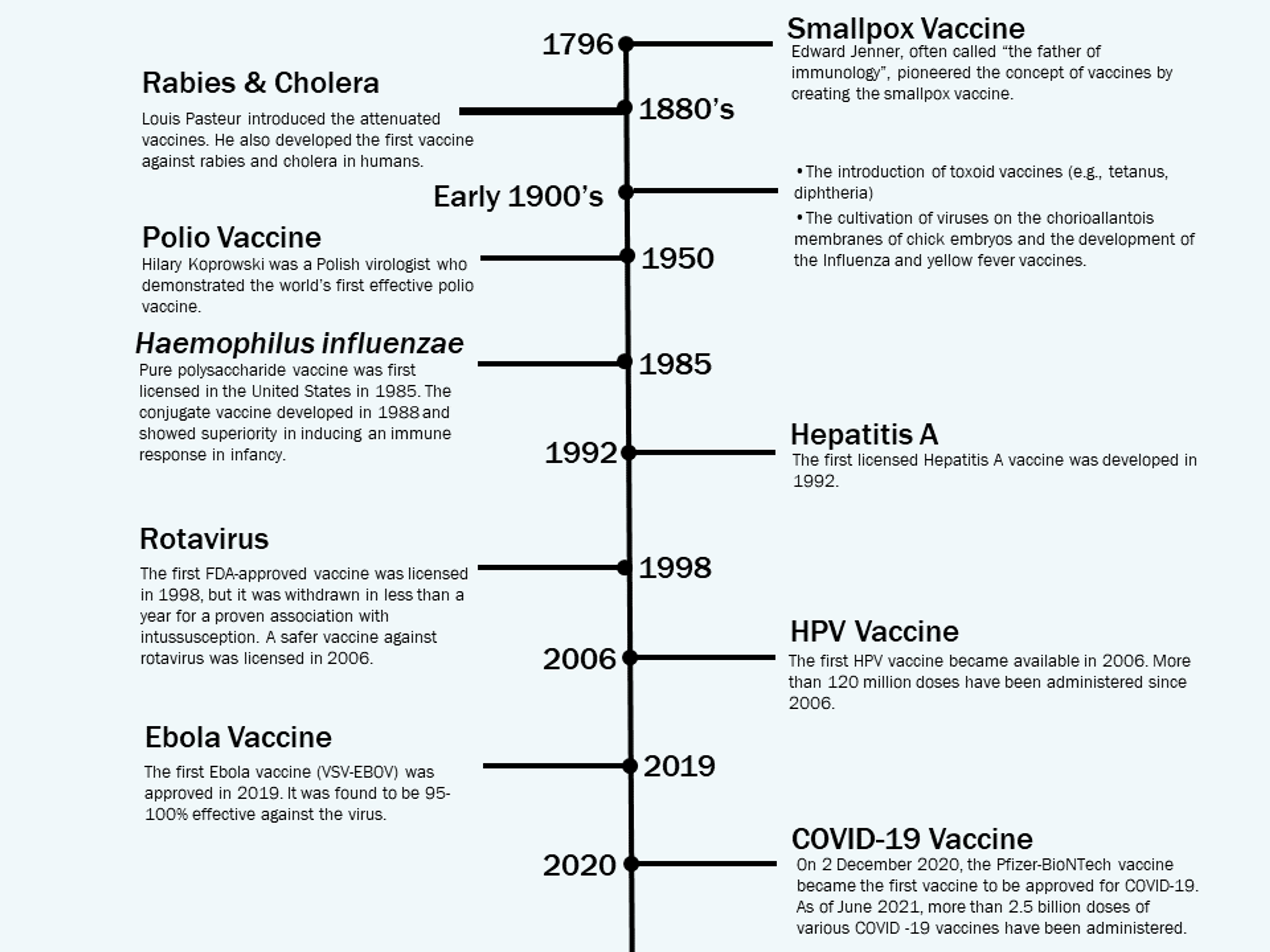
Cureus Vaccine Development Throughout History

With Fda Verging On Its First Covid Booster Approval Pfizer And Moderna Will Have To Recalibrate Revenues Again Fiercepharma

Fda Approval Updates Pfizer Moderna And J J Covid 19 Vaccine Goodrx

Clover Biopharmaceuticals Science Trimer Tag
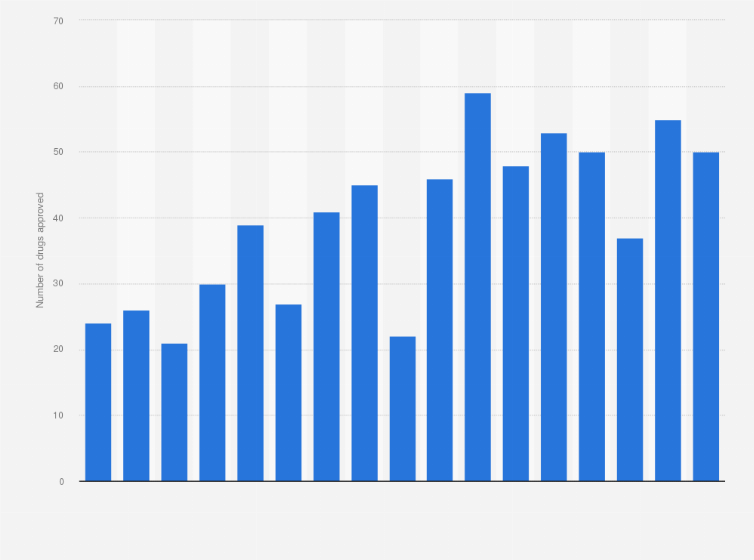
Cder Drug Approvals U S 2008 2020 Statista
Https Www Fda Gov Media 135078 Download

Pfizer Biontech Covid 19 Vaccine

Covid 19 Vaccine Information Camden County Nj

Pfizer Seeks Full Fda Approval For Covid 19 Vaccine Coronavirus Updates Npr
Can You Get The Moderna Covid 19 Vaccine If You Re Immunocompromised

Pfizer Biontech Covid 19 Vaccine
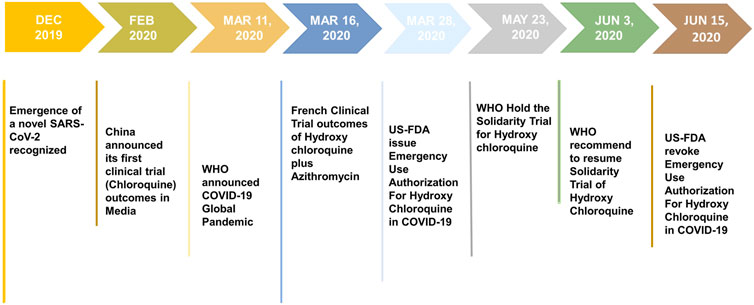
Frontiers The Rise And Fall Of Chloroquine Hydroxychloroquine As Compassionate Therapy Of Covid 19 Pharmacology


Post a Comment for "Fda Approved Vaccines 2019"